Search
- Page Path
- HOME > Search
- Diabetes, obesity and metabolism
- Greater Severity of Steatosis Is Associated with a Higher Risk of Incident Diabetes: A Retrospective Longitudinal Study
- Ji Min Han, Jung Hwan Cho, Hye In Kim, Sunghwan Suh, Yu-Ji Lee, Jung Won Lee, Kwang Min Kim, Ji Cheol Bae
- Endocrinol Metab. 2023;38(4):418-425. Published online July 12, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1729
- 1,058 View
- 77 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Fatty liver is associated with increased risk of developing type 2 diabetes. We aimed to evaluate whether the severity of hepatic steatosis is associated with incident diabetes.
Methods
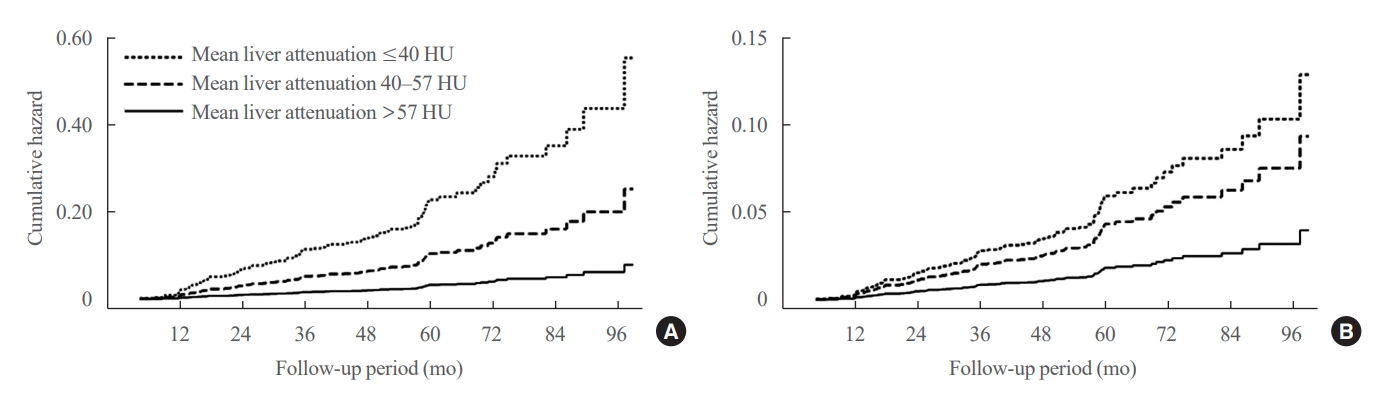
We conducted a longitudinal analysis using data from 1,798 participants who underwent a comprehensive health checkup and abdominal computed tomography (CT). We assessed the association between baseline liver attenuation value on non-contrast CT images and risk of incident diabetes. All the participants were categorized into three groups based on the baseline liver attenuation value on non-contrast CT images: without hepatic steatosis (>57 Hounsfield unit [HU]), mild hepatic steatosis (41–57 HU), and moderate to severe hepatic steatosis (≤40 HU).
Results
During a median follow-up period of 5 years, 6.0% of the study participants progressed to diabetes. The incidence of diabetes was 17.3% in the moderate to severe hepatic steatosis group, 9.0% in the mild steatosis group, and 2.9% in those without hepatic steatosis. In a multivariate adjustment model, as compared with participants without hepatic steatosis, those with moderate to severe steatosis had a hazard ratio (HR) of 3.24 (95% confidence interval [CI], 1.64 to 4.2) for the development of diabetes, and those in the mild steatosis group had a HR of 2.33 (95% CI, 1.42 to 3.80). One standard deviation decrease in mean CT attenuation values of the liver was associated with a 40% increase in the development of diabetes (multivariate adjusted HR, 1.40; 95% CI, 1.2 to 1.63).
Conclusion
We found a positive association between severity of hepatic steatosis and risk of incident diabetes. Greater severity of steatosis was associated with a higher risk of incident diabetes.

- Diabetes
- Pioglitazone Attenuates Palmitate-Induced Inflammation and Endoplasmic Reticulum Stress in Pancreatic β-Cells
- Seok-Woo Hong, Jinmi Lee, Jung Hwan Cho, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Cheol-Young Park, Ki-Won Oh, Sung-Woo Park, Won-Young Lee
- Endocrinol Metab. 2018;33(1):105-113. Published online March 21, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.1.105
- 6,256 View
- 96 Download
- 19 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background The nuclear receptor peroxisome proliferator-activator gamma (PPARγ) is a useful therapeutic target for obesity and diabetes, but its role in protecting β-cell function and viability is unclear.
Methods To identify the potential functions of PPARγ in β-cells, we treated mouse insulinoma 6 (MIN6) cells with the PPARγ agonist pioglitazone in conditions of lipotoxicity, endoplasmic reticulum (ER) stress, and inflammation.
Results Palmitate-treated cells incubated with pioglitazone exhibited significant improvements in glucose-stimulated insulin secretion and the repression of apoptosis, as shown by decreased caspase-3 cleavage and poly (adenosine diphosphate [ADP]-ribose) polymerase activity. Pioglitazone also reversed the palmitate-induced expression of inflammatory cytokines (tumor necrosis factor α, interleukin 6 [IL-6], and IL-1β) and ER stress markers (phosphor-eukaryotic translation initiation factor 2α, glucose-regulated protein 78 [GRP78], cleaved-activating transcription factor 6 [ATF6], and C/EBP homologous protein [CHOP]), and pioglitazone significantly attenuated inflammation and ER stress in lipopolysaccharide- or tunicamycin-treated MIN6 cells. The protective effect of pioglitazone was also tested in pancreatic islets from high-fat-fed KK-Ay mice administered 0.02% (wt/wt) pioglitazone or vehicle for 6 weeks. Pioglitazone remarkably reduced the expression of ATF6α, GRP78, and monocyte chemoattractant protein-1, prevented α-cell infiltration into the pancreatic islets, and upregulated glucose transporter 2 (Glut2) expression in β-cells. Moreover, the preservation of β-cells by pioglitazone was accompanied by a significant reduction of blood glucose levels.
Conclusion Altogether, these results support the proposal that PPARγ agonists not only suppress insulin resistance, but also prevent β-cell impairment via protection against ER stress and inflammation. The activation of PPARγ might be a new therapeutic approach for improving β-cell survival and insulin secretion in patients with diabetes mellitus
-
Citations
Citations to this article as recorded by- Nr1h4 and Thrb ameliorate ER stress and provide protection in the MPTP mouse model of Parkinson’s
Nancy Ahuja, Shalini Gupta, Rashmi Arora, Ella Bhagyaraj, Drishti Tiwari, Sumit Kumar, Pawan Gupta
Life Science Alliance.2024; 7(7): e202302416. CrossRef - Prosthetic vascular grafts engineered to combat calcification: Progress and future directions
Taylor K. Brown, Sara Alharbi, Karen J. Ho, Bin Jiang
Biotechnology and Bioengineering.2023; 120(4): 953. CrossRef - Obesity, diabetes mellitus, and cardiometabolic risk: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2023
Harold Edward Bays, Shagun Bindlish, Tiffany Lowe Clayton
Obesity Pillars.2023; 5: 100056. CrossRef - Metformin promotes osteogenic differentiation and prevents hyperglycaemia-induced osteoporosis by suppressing PPARγ expression
Lifeng Zheng, Ximei Shen, Yun Xie, Hong Lian, Sunjie Yan, Shizhong Wang
Acta Biochimica et Biophysica Sinica.2023; 55(3): 394. CrossRef - Peroxisome proliferator-activated receptors as targets to treat metabolic diseases: Focus on the adipose tissue, liver, and pancreas
Henrique Souza-Tavares, Carolline Santos Miranda, Isabela Macedo Lopes Vasques-Monteiro, Cristian Sandoval, Daiana Araujo Santana-Oliveira, Flavia Maria Silva-Veiga, Aline Fernandes-da-Silva, Vanessa Souza-Mello
World Journal of Gastroenterology.2023; 29(26): 4136. CrossRef - Nicotinamide N-methyltransferase upregulation contributes to palmitate-elicited peroxisome proliferator-activated receptor transactivation in hepatocytes
Qing Song, Jun Wang, Alexandra Griffiths, Samuel Man Lee, Iredia D. Iyamu, Rong Huang, Jose Cordoba-Chacon, Zhenyuan Song
American Journal of Physiology-Cell Physiology.2023; 325(1): C29. CrossRef - The global perspective on peroxisome proliferator-activated receptor γ (PPARγ) in ectopic fat deposition: A review
Yanhao Qiu, Mailin Gan, Xingyu Wang, Tianci Liao, Qiuyang Chen, Yuhang Lei, Lei Chen, Jinyong Wang, Ye Zhao, Lili Niu, Yan Wang, Shunhua Zhang, Li Zhu, Linyuan Shen
International Journal of Biological Macromolecules.2023; 253: 127042. CrossRef - Chemical inducer of regucalcin attenuates lipopolysaccharide‐induced inflammatory responses in pancreatic MIN6 β‐cells and RAW264.7 macrophages
Tomiyasu Murata, Kazunori Hashimoto, Susumu Kohno, Chiaki Takahashi, Masayoshi Yamaguchi, Chihiro Ito, Itoigawa Masataka, Roji Kojima, Kiyomi Hikita, Norio Kaneda
FEBS Open Bio.2022; 12(1): 175. CrossRef - Targets for rescue from fatty acid-induced lipotoxicity in pancreatic beta cells
Seok-Woo Hong, Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2022; 4(2): 57. CrossRef - Analysis of changes in the proteomic profile of porcine corpus luteum during different stages of the oestrous cycle: effects of PPAR gamma ligands
Zuzanna Kunicka, Karol Mierzejewski, Aleksandra Kurzyńska, Robert Stryiński, Jesús Mateos, Mónica Carrera, Monika Golubska, Iwona Bogacka, Xiaolong Wang
Reproduction, Fertility and Development.2022; 34(11): 776. CrossRef - Activation of PPARγ Protects Obese Mice from Acute Lung Injury by Inhibiting Endoplasmic Reticulum Stress and Promoting Mitochondrial Biogenesis
Yin Tang, Ke Wei, Ling Liu, Jingyue Ma, Siqi Wu, Wenjing Tang, Stéphane Mandard
PPAR Research.2022; 2022: 1. CrossRef - Effect of Pioglitazone on endoplasmic reticulum stress regarding in situ perfusion rat model
Vivien Telek, Luca Erlitz, Ibitamuno Caleb, Tibor Nagy, Mónika Vecsernyés, Bálint Balogh, György Sétáló, Péter Hardi, Gábor Jancsó, Ildikó Takács
Clinical Hemorheology and Microcirculation.2021; 79(2): 311. CrossRef - Inflammation in Metabolic Diseases and Insulin Resistance
Won-Young Lee
Cardiovascular Prevention and Pharmacotherapy.2021; 3(2): 31. CrossRef - Current Status of Endoplasmic Reticulum Stress in Type II Diabetes
Sagir Mustapha, Mustapha Mohammed, Ahmad Khusairi Azemi, Abubakar Ibrahim Jatau, Aishatu Shehu, Lukman Mustapha, Ibrahim Muazzamu Aliyu, Rabi’u Nuhu Danraka, Abdulbasit Amin, Auwal Adam Bala, Wan Amir Nizam Wan Ahmad, Aida Hanum Ghulam Rasool, Mohd Rais M
Molecules.2021; 26(14): 4362. CrossRef - JunD Regulates Pancreatic β-Cells Function by Altering Lipid Accumulation
Kexin Wang, Yixin Cui, Peng Lin, Zhina Yao, Yu Sun
Frontiers in Endocrinology.2021;[Epub] CrossRef - Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial
Giuseppe Della Pepa, Marco Russo, Marilena Vitale, Fabrizia Carli, Claudia Vetrani, Maria Masulli, Gabriele Riccardi, Olga Vaccaro, Amalia Gastaldelli, Angela A. Rivellese, Lutgarda Bozzetto
Diabetes Research and Clinical Practice.2021; 178: 108984. CrossRef - Radioprotective Effect of Pioglitazone Against Genotoxicity Induced by Ionizing Radiation in Healthy Human Lymphocytes
Roya Kazemi, Seyed J. Hosseinimehr
Cardiovascular & Hematological Agents in Medicinal Chemistry .2021; 19(1): 72. CrossRef - Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes
Maria Lytrivi, Anne-Laure Castell, Vincent Poitout, Miriam Cnop
Journal of Molecular Biology.2020; 432(5): 1514. CrossRef - Artemisinin and dihydroartemisinin promote β-cell apoptosis induced by palmitate via enhancing ER stress
Ke Chen, Hu Hua, Ziyang Zhu, Tong Wu, Zhanjun Jia, Qianqi Liu
Apoptosis.2020; 25(3-4): 192. CrossRef - Mechanisms of impaired pancreatic β‑cell function in high‑fat diet‑induced obese mice: The role of endoplasmic reticulum stress
Xiaoqing Yi, Xuan Cai, Sisi Wang, Yanfeng Xiao
Molecular Medicine Reports.2020;[Epub] CrossRef - Docosahexaenoic and Eicosapentaenoic Acids Prevent Altered-Muc2 Secretion Induced by Palmitic Acid by Alleviating Endoplasmic Reticulum Stress in LS174T Goblet Cells
Quentin Escoula, Sandrine Bellenger, Michel Narce, Jérôme Bellenger
Nutrients.2019; 11(9): 2179. CrossRef - PPAR-γ agonist, pioglitazone, reduced oxidative and endoplasmic reticulum stress associated with L-NAME-induced hypertension in rats
Eman Soliman, Shereen F. Behairy, Nabila N. El-maraghy, Shimaa M. Elshazly
Life Sciences.2019; 239: 117047. CrossRef - Changes of MODY signal pathway genes in the endoplasmic reticulum stress in INS-1-3 cells
Yanan Dong, Shirui Li, Wenhui Zhao, Yanlei Wang, Tingting Ge, Jianzhong Xiao, Yukun Li, Herve Le Stunff
PLOS ONE.2018; 13(6): e0198614. CrossRef
- Nr1h4 and Thrb ameliorate ER stress and provide protection in the MPTP mouse model of Parkinson’s

- Diabetes
- The Association between Persistent Hypertriglyceridemia and the Risk of Diabetes Development: The Kangbuk Samsung Health Study
- Yu Hyun Kwon, Seul-Ki Kim, Jung Hwan Cho, Hyemi Kwon, Se Eun Park, Hyung-Geun Oh, Cheol-Young Park, Won-Young Lee, Ki-Won Oh, Sung-Woo Park, Eun-Jung Rhee
- Endocrinol Metab. 2018;33(1):55-61. Published online January 30, 2018
- DOI: https://doi.org/10.3803/EnM.2018.33.1.55
- 4,175 View
- 62 Download
- 13 Web of Science
- 14 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub Background Hypertriglyceridemia is known to have an association with increased risks of insulin resistance and diabetes. The aim of this study was to investigate the risk of diabetes mellitus, according to changes in the concentrations of triglycerides, over time.
Methods A total of 15,932 non-diabetic participants (mean age 43.2 years, 68% men) who attended five consecutive annual health check-ups at Kangbuk Samsung Hospital, between January 2010 and December 2014, were recruited. Participants were classified according to their triglyceride concentrations; normal (<150 mg/dL) and abnormal (≥150 mg/dL). According to the triglyceride levels in 2010 and 2012, subjects were divided into four groups: normal-normal, normal-abnormal, abnormal-normal, and abnormal-abnormal. The risk for incident diabetes was assessed in 2014.
Results Among the total subjects, 67.5% belonged to the normal-normal group, 8.6% to the normal-abnormal group, 9.4% to the abnormal-normal group, and 14.5% to the abnormal-abnormal group. A total of 234 subjects (1.5%) were newly diagnosed with diabetes, between 2010 and 2014. Over 4 years, 1%, 1.5%, 2.1%, and 3.0% of the subjects developed diabetes in the normal-normal, normal-abnormal, abnormal-normal, and abnormal-abnormal groups, respectively. When the risk for incident diabetes was analyzed in the groups, after adjusting the confounding variables, a 1.58-fold increase in the risk of diabetes (95% confidence interval [CI], 1.10 to 2.26) was observed in the participants with persistent hypertriglyceridemia (abnormal-abnormal group). This was attenuated by further adjustments for body mass index (BMI) (hazard ratio, 1.25; 95% CI, 0.86 to 1.80).
Conclusion In this large study population, persistent hypertriglyceridemia, over a period of 2 years, was significantly associated with the risk of incident diabetes, which was attenuated after adjustment for BMI.
-
Citations
Citations to this article as recorded by- Cumulative exposure to hypertriglyceridemia and risk of type 2 diabetes in young adults
Min-Kyung Lee, Kyungdo Han, Bongsung Kim, Jong-Dai Kim, Moon Jung Kim, Byungpyo Kim, Jung Heo, Jiyeon Ahn, Seo-Young Sohn, Jae-Hyuk Lee
Diabetes Research and Clinical Practice.2024; 208: 111109. CrossRef - Usefulness of SPISE Index for Screening and Detection of Early Stages of Insulin Resistance among Chilean Young Adults
Isabel Pereyra González, Sandra Lopez-Arana
Annals of Nutrition and Metabolism.2023; 79(4): 372. CrossRef - Lipid variability in patients with diabetes mellitus
Jeongmin Lee, Seung-Hwan Lee
Cardiovascular Prevention and Pharmacotherapy.2023; 5(4): 126. CrossRef - Sesamin: A Promising Therapeutic Agent for Ameliorating Symptoms of Diabetes
Shu-Ming Huang, Cheng-Hung Chuang, Christine Joyce F. Rejano, Lemmuel L. Tayo, Cheng-Yang Hsieh, Steven Kuan-Hua Huang, Po-Wei Tsai
Molecules.2023; 28(21): 7255. CrossRef - Variability, Mean, and Baseline Values of Metabolic Parameters in Predicting Risk of Type 2 Diabetes
Duong Duc Pham, Jaekyung Song, Yunwan Jeon, Ibrahimi Hajar, Chae Hun Leem
The Journal of Clinical Endocrinology & Metabolism.2022; 107(5): 1270. CrossRef - Lipid Variability and Diabetes Mellitus
Jeongmin Lee, Seung-Hwan Lee
The Journal of Korean Diabetes.2022; 23(1): 28. CrossRef - The Risk of Type 2 Diabetes Mellitus in a Russian Population Cohort According to Data from the HAPIEE Project
Svetlana V. Mustafina, Oksana D. Rymar, Liliya V. Shcherbakova, Evgeniy G. Verevkin, Hynek Pikhart, Olga V. Sazonova, Yuliya I. Ragino, Galina I. Simonova, Martin Bobak, Sofia K. Malyutina, Mikhail I. Voevoda
Journal of Personalized Medicine.2021; 11(2): 119. CrossRef - The influence of VDR polymorphisms on the type 2 diabetes susceptibility in Chinese: an interaction with hypertriglyceridemia
Dongdong Zhang, Cheng Cheng, Yan Wang, Yuan Xue, Yaping Liu, Yiming Liu, Mingming Feng, Ze Xu, Wenjie Li, Xing Li
Molecular Genetics and Genomics.2021; 296(4): 837. CrossRef - Development and validation of a new diabetes index for the risk classification of present and new-onset diabetes: multicohort study
Shinje Moon, Ji-Yong Jang, Yumin Kim, Chang-Myung Oh
Scientific Reports.2021;[Epub] CrossRef - Hypertriglyceridemia as an Independent Predictor for Ten-Year Incidence of Diabetes in Thais
Suranut Charoensri, Supatida Turnsaket, Chatlert Pongchaiyakul
Vascular Health and Risk Management.2021; Volume 17: 519. CrossRef - Prevalence and Current Management of Cardiovascular Risk Factors in Korean Adults Based on Fact Sheets
Eun-Jung Rhee
Endocrinology and Metabolism.2020; 35(1): 85. CrossRef - HDL-Cholesterol, Its Variability, and the Risk of Diabetes: A Nationwide Population-Based Study
Seung-Hwan Lee, Hun-Sung Kim, Yong-Moon Park, Hyuk-Sang Kwon, Kun-Ho Yoon, Kyungdo Han, Mee Kyoung Kim
The Journal of Clinical Endocrinology & Metabolism.2019; 104(11): 5633. CrossRef - Response: The Association between Persistent Hypertriglyceridemia and the Risk of Diabetes Development: The Kangbuk Samsung Health Study (Endocrinol Metab 2018;33:55–61, Yu Hyun Kwon et al.)
Eun-Jung Rhee, Yu Hyun Kwon
Endocrinology and Metabolism.2018; 33(3): 425. CrossRef - The Association between Persistent Hypertriglyceridemia and the Risk of Diabetes Development: The Kangbuk Samsung Health Study (Endocrinol Metab 2018;33:55–61, Yu Hyun Kwon et al.)
Mi Hae Seo
Endocrinology and Metabolism.2018; 33(2): 305. CrossRef
- Cumulative exposure to hypertriglyceridemia and risk of type 2 diabetes in young adults

- Clinical Study
- Changes in Body Composition According to Age and Sex among Young Non-Diabetic Korean Adults: The Kangbuk Samsung Health Study
- Seul-Ki Kim, Yu-Hyun Kwon, Jung Hwan Cho, Da Young Lee, Se Eun Park, Hyung-Geun Oh, Cheol-Young Park, Won-Young Lee, Ki-Won Oh, Sung-Woo Park, Eun-Jung Rhee
- Endocrinol Metab. 2017;32(4):442-450. Published online November 21, 2017
- DOI: https://doi.org/10.3803/EnM.2017.32.4.442
- 6,187 View
- 63 Download
- 18 Web of Science
- 20 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Age-related decreases in lean mass represent a serious health problem. We aimed to analyze the risks of rapid decreases in lean mass by age and sex in relatively young Korean adults during a 4-year follow-up study.
Methods A total of 65,856 non-diabetic participants (59.5% men, mean age 39.1 years) in a health screening program were subjected to bioimpedance body composition analyses and metabolic parameter analyses at baseline and after 4 years. The participants were sub-divided according to age, and additionally to six groups by age and the degree of body weight change over the 4-year period. The actual changes in body weight, lean mass, and fat mass and the percent changes over the 4-year period were assessed.
Results The percent change in lean mass decreased and the percent change of fat mass increased with increasing age in every age and sex group. However, the annual percent decrease in lean mass and percent increase in fat mass were significantly higher among women than among men (−0.26% vs. −0.15% and 0.34% vs. 0.42%, respectively;
P <0.01). Participants who were older than 50 years and had a weight loss <−5% during the 4 years had significantly greater decreases in lean mass and smaller decreases in fat mass, compared to those who were younger than 50 years. An odds ratio analysis to determine the lowest quartile of the percent change in lean mass according to age group revealed that participants older than 60 years had a significantly increased risk of a rapid decrease in the lean mass percentage (2.081; 95% confidence interval, 1.678 to 2.581).Conclusion Even in this relatively young study population, the lean mass decreased significantly with age, and the risk of a rapid decrease in lean mass was higher among women than among men. Furthermore, the elderly exhibited a significantly more rapid decrease in lean mass, compared with younger participants.
-
Citations
Citations to this article as recorded by- Obesity, Physical Performance, Balance Confidence, and Falls in Community-Dwelling Older Adults: Results from the Korean Frailty and Aging Cohort Study
Ga Yang Shim, Myung Chul Yoo, Yunsoo Soh, Jinmann Chon, Chang Won Won
Nutrients.2024; 16(5): 614. CrossRef - Multisystem physiological perspective of human frailty and its modulation by physical activity
Joseph A. Taylor, Paul L. Greenhaff, David B. Bartlett, Thomas A. Jackson, Niharika A. Duggal, Janet M. Lord
Physiological Reviews.2023; 103(2): 1137. CrossRef - Partial weight reduction protocols in cats lead to better weight outcomes, compared with complete protocols, in cats with obesity
Alexander J. German, Georgiana R. T. Woods-Lee, Vincent Biourge, John Flanagan
Frontiers in Veterinary Science.2023;[Epub] CrossRef - Multifaceted effects of obesity on cancer immunotherapies: Bridging preclinical models and clinical data
Logan V. Vick, Robert J. Canter, Arta M. Monjazeb, William J. Murphy
Seminars in Cancer Biology.2023; 95: 88. CrossRef - Age-Related Trends in Body Composition among Women Aged 20–80 Years: A Cross-Sectional Study
Nirmala Rathnayake, Hasanga Rathnayake, Sarath Lekamwasam, Aron Weller
Journal of Obesity.2022; 2022: 1. CrossRef - Increased Consumption of Unsaturated Fatty Acids Improves Body Composition in a Hypercholesterolemic Chinese Population
Sumanto Haldar, Shalini Ponnalagu, Farhana Osman, Shia Lyn Tay, Long Hui Wong, Yuan Rong Jiang, Melvin Khee Shing Leow, Christiani Jeyakumar Henry
Frontiers in Nutrition.2022;[Epub] CrossRef - Development and Evaluation of a Low-cost Dairy Food Supplement with Mauritia Flexuosa (Buriti) to Combat Malnutrition: Translational Study in Mice and Institutionalized Elderly Woman
Audrey Handyara Bicalho, Fabio Ribeiro Santos, Daniele Cristina Moreira, Victor Hugo Dantas Guimarães, Guilherme Henrique Ribeiro, Alfredo Mauricio Batista De Paula, André Luis Sena Guimarães, Ulisses A. Pereira, Theles Costa, Caroline Liboreiro Paiva, Ma
Current Aging Science.2022; 15(1): 37. CrossRef - The missense variant, rs373863828, in CREBRF plays a role in longitudinal changes in body mass index in Samoans
Haoyi Fu, Nicola L. Hawley, Jenna C. Carlson, Emily M. Russell, Alysa Pomer, Hong Cheng, Take Naseri, Muagututi‘a Sefuiva Reupena, Ranjan Deka, Courtney C. Choy, Stephen T. McGarvey, Ryan L. Minster, Daniel E. Weeks
Obesity Research & Clinical Practice.2022; 16(3): 220. CrossRef - Relationship Between Handgrip Strength and the Prevalence of Diabetes Mellitus Among Korean Adults: Korean National Health and Nutrition Examination Survey, 2014-2018
Sung-hyun Hong, Ji-yong Byeon, Ji-hee Min, Dong-hyuk Park, Won-hee Cho, Justin Y. Jeon
Exercise Science.2021; 30(1): 110. CrossRef - Cutoff points of adiposity anthropometric indices for low muscle mass screening in middle-aged and older healthy women
Rafaela Andrade do Nascimento, Mariana Carmem Apolinário Vieira, Rafaella Silva dos Santos Aguiar Gonçalves, Mayle Andrade Moreira, Maria Socorro Medeiros de Morais, Saionara Maria Aires da Câmara, Álvaro Campos Cavalcanti Maciel
BMC Musculoskeletal Disorders.2021;[Epub] CrossRef - Edema-like symptoms are common in ultra-distance cyclists and driven by overdrinking, use of analgesics and female sex – a study of 919 athletes
Philipp Gauckler, Jana S. Kesenheimer, Andreas Kronbichler, Fiona R. Kolbinger
Journal of the International Society of Sports Nutrition.2021;[Epub] CrossRef - Prevalence of low muscle mass and associated factors in community-dwelling older adults in Singapore
Siew Ling Tey, Dieu Thi Thu Huynh, Yatin Berde, Geraldine Baggs, Choon How How, Yen Ling Low, Magdalin Cheong, Wai Leng Chow, Ngiap Chuan Tan, Samuel Teong Huang Chew
Scientific Reports.2021;[Epub] CrossRef - Effects of low skeletal muscle mass and sarcopenic obesity on albuminuria: a 7-year longitudinal study
Jee Hee Yoo, Gyuri Kim, Sung Woon Park, Min Sun Choi, Jiyeon Ahn, Sang-Man Jin, Kyu Yeon Hur, Moon-Kyu Lee, Mira Kang, Jae Hyeon Kim
Scientific Reports.2020;[Epub] CrossRef - Age group and gender-wise comparison of obesity indices in subjects of Varanasi
Kumar Sarvottam, Prabhat Ranjan, Umashree Yadav
Indian Journal of Physiology and Pharmacology.2020; 64: 109. CrossRef - DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non-Atopic Asthma
Ayoung Jeong, Medea Imboden, Akram Ghantous, Alexei Novoloaca, Anne-Elie Carsin, Manolis Kogevinas, Christian Schindler, Gianfranco Lovison, Zdenko Herceg, Cyrille Cuenin, Roel Vermeulen, Deborah Jarvis, André Amaral, Florian Kronenberg, Paolo Vineis, Nic
International Journal of Environmental Research and Public Health.2019; 16(4): 600. CrossRef - Body shape, fear of falling, physical performance, and falls among individuals aged 55 years and above
Sheng Hui Kioh, Sumaiyah Mat, Shahrul B. Kamaruzzaman, Fatimah Ibrahim, Mas Sahidayana Mokhtar, Noran N. Hairi, Robert G. Cumming, Phyo Kyaw Myint, Maw Pin Tan
European Geriatric Medicine.2019; 10(5): 801. CrossRef - Low lean tissue mass can be a predictor of one-year survival in hemodialysis patients
Aleksandra Rymarz, Julia Gibińska, Maria Zajbt, Wiesław Piechota, Stanisław Niemczyk
Renal Failure.2018; 40(1): 231. CrossRef - Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7‐Year Longitudinal Study
Gyuri Kim, Seung‐Eun Lee, You‐Bin Lee, Ji Eun Jun, Jiyeon Ahn, Ji Cheol Bae, Sang‐Man Jin, Kyu Yeon Hur, Jae Hwan Jee, Moon‐Kyu Lee, Jae Hyeon Kim
Hepatology.2018; 68(5): 1755. CrossRef - Association between abdominal obesity and increased risk for the development of hypertension regardless of physical activity: A nationwide population‐based study
Eun‐Jung Rhee, Jung‐Hwan Cho, Hyemi Kwon, Se‐Eun Park, Jin‐Hyung Jung, Kyung‐Do Han, Yong‐Gyu Park, Hye Soon Park, Yang‐Hyun Kim, Soon‐Jib Yoo, Won‐Young Lee
The Journal of Clinical Hypertension.2018; 20(10): 1417. CrossRef - Decreasing Lean Body Mass with Age: Challenges and Opportunities for Novel Therapies
Chrysoula Boutari, Christos S. Mantzoros
Endocrinology and Metabolism.2017; 32(4): 422. CrossRef
- Obesity, Physical Performance, Balance Confidence, and Falls in Community-Dwelling Older Adults: Results from the Korean Frailty and Aging Cohort Study


 KES
KES

 First
First Prev
Prev



